|
Listen to Post
|
Listen to this article now:
The EU MDR is the European Union Medical Device Regulation 2017/745 that was released in 2017 by the European Parliament and the Council of the European Union and entered into application on 26 May 2021. The intent of the EU MDR regulation is to ensure a high standard of safety and quality for medical devices that are produced in, or supplied to, member countries of the European Union. In this article we will explore how the EU MDR affects the translation process of medical devices.
MDR creates translation challenges
Preciseness is the hallmark of excellence in medical practice. In the business of life-saving, there is little room for error.
Accurate translations of brochures, user manuals, and other information accompanying medical devices are therefore as critical to success as the accuracy of the device itself. To that end, the MDR requires medical device manufacturers who want to distribute their products within the EU to include accurate translations for each member nation.
Article 10 (section 11) of the MDR states, “Manufacturers shall ensure that the device is accompanied by the information…in an official Union language(s) determined by the Member State in which the device is made available to the user or patient.” Medical device and other life science companies, therefore, need to conduct a language gap analysis to determine if any of the EU member state languages are missing.
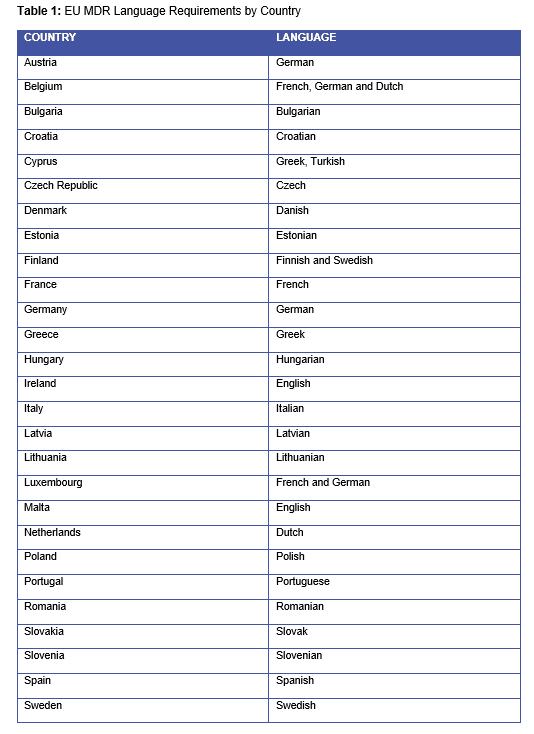
If so, these companies need a strategy to add those missing languages so that they can distribute their product in those countries. Table 1, below, shows the massive number of languages that device makers need to include.
MDR’s impact on the translation process
The MDR expanded the scope of what it terms “medical devices” to include “any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in combination, for human beings.” It includes diagnostic tools, surgical and exploratory instruments, and devices that facilitate healing, repair, or restoration.
The new regulation’s scope now includes “bandages, splints, and…hospital beds,” as well as cosmetic devices like “non-prescription colored content lenses, dermal fillers, and equipment used for cosmetic procedures like liposuction.”
The EU MDR puts the onus on manufacturers, rather than their member states, to comply with the translation requirements. These requirements include:
-Translations must be available in all EU languages: Accompanying every device must be a translation of all required documentation in each of the 24 official EU languages.
-Many CE approvals require a translation before the approval process begins: In the past, medical devices needed only to provide translations after a device earned the CE mark. However, many documents required for the new approval process, such as Instructions for Use (IFUs), now require translations into all 24 languages.
-Translations and original language must use clear, precise wording: Not only must labels, safety instructions, and IFUs have complete clarity in the translated material, but the original source’s language must also be crystal-clear.
-Medical device documents must be compatible with the EU database: Since all the data that pertain to medical devices will eventually be stored in EUDAMED, the EU’s central medical database, manufacturers must ensure that their translators use compatible content management systems (CMS) that integrate seamlessly with their own translation management system. The time to implement these new translation guidelines soon approaches. Rather than “pulling back from EU markets,” like some medical device manufacturers planned to do, there is a better option.
Expand translation’s role to ensure MDR compliance
Translation companies have a wealth of tools to streamline the MDR compliance process across a broad range of applications. These tools include:
-Computer-assisted translation tools: Computer-assisted translation tools (CATs) reuse previously translated content to support new translations. These tools ensure consistency across both newer and older content.
-Machine translation solutions: Machine translation (MT) tools boost translation speed and reduce costs. These AI-powered tools are an excellent solution for medical device manufacturers who need to translate legacy files.
-API connectors: API connectors streamline the translation process by integrating technologies between the medical device company and the translation provider.
Telehealth: translation companies’ expanded role in the Covid-19 era
One of the most critical needs that emerged during the pandemic was access to no-contact or low-contact diagnosis and treatment. During past pandemics, that ability was the stuff of science fiction. However, with the advent of groundbreaking technologies, healthcare providers now have the tools to adopt telehealth solutions to diagnose and treat their patients remotely.
Challenges in remote patient care
This breakthrough does bring with it some challenges in the legal arena. Although governments “have worked swiftly to ease restriction on telemedicine,” as Gary Giampetruzzi et al. point out, legal risks, including abuse and fraud, have entered the picture. Patient consent and operating instructions for prescribed devices also run the risk of a lack of clarity during remote calls.
Those risks run higher yet when providers live in a country with two or more official languages, such as Cyprus, Belgium, and Luxembourg. In addition, countries with high numbers of immigrants, such as Germany – with its three million ethnic Turks, some of them still struggling to speak German – also face these risks
Translation’s role in medical device software and app localization
With the shift to telemedicine and the need to collaborate with medical professionals all over the EU and elsewhere, medical device companies need to localize the software and mobile applications that provide service and support for their devices.
Translators can ensure that website and mobile app text, buttons, fields, and user guides align with each other in each of the EU languages. With linguistic and functional testing, they can ensure that users will enjoy a seamless user experience in their native tongue.
Translation’s role in digitizing the medical device model
As have most industries, medtech companies have expanded their digital footprint during the COVID-19 crisis. Whether it’s digital detailing, digital media, or even digital device demonstrations, translation has a role to play in a global environment.
As Siddhartha Chadha et al. point out in their April 22, 2020 McKinsey article, the global pandemic has driven medtech companies to partner with companies outside their sector to meet the demand for their products and services. Translators number among these partners, innovating new methods of digital support for their devices.

Supplemented by accurate translation, digitization facilitates cooperation
Remote management software plays a large role in medtech companies’ digital innovation. In cooperation with healthcare professionals, medical device manufacturers are creating and promoting remote options for screenings and monitoring.
From making apps to offer people with diabetes the ability to check their A1C levels to creating videos that explain new devices to hospital stakeholders and healthcare providers, translation can be the key that unlocks new global doors for device makers.
App and software localization helps, but with 65 percent of the world’s population being visual learners, video communications are a must when in-person demonstrations are impossible. Translation companies can provide the multilingual voiceovers and subtitles that make these videos come alive in the audience’s minds.
All across the board, translation has a significant role to play as medical device companies deal with the challenges that the global pandemic has brought with it.
Looking for a dependable provider for accurate, rapid translation services to help you achieve MDR compliance? Contact the friendly team at Commit Global today!