Life Sciences Translation Services
Balancing the triangle of accuracy, confidentiality and timely delivery in Life Sciences & Healthcare
Life Sciences Translations by Subject Matter Experts
When the safety of a patient depends on the understanding of directions and the correct operation of medical devices, translation requires precision and up-to-date technical knowledge. Whether you are localizing medical device documentation, translating patient data or preparing clinical research related materials, our Life Sciences Localization team produces high quality and accurate medical translations that ensure patients’ safety is not put at risk.
Because of the highly technical, sensitive and regulated nature of medical texts, Commit Global fully understands the need for
- accuracy
- confidentiality
- timely delivery

and responds by:
- selecting only professional native medical translators supported by subject-matter experts who provide real-time consulting per specific medical field
- using the ISO 13485 quality management system in our translation process to prevent misuse and ensure that your medical devices are safe for their intended purpose.
- implementing ISO 27001 certified processes to safeguard your sensitive and confidential information
- working on a “follow the sun” model, having established teams across continents who respond during your time zone and ensure project workflow runs smoothly across time zones without compromising quality
We can help you with:
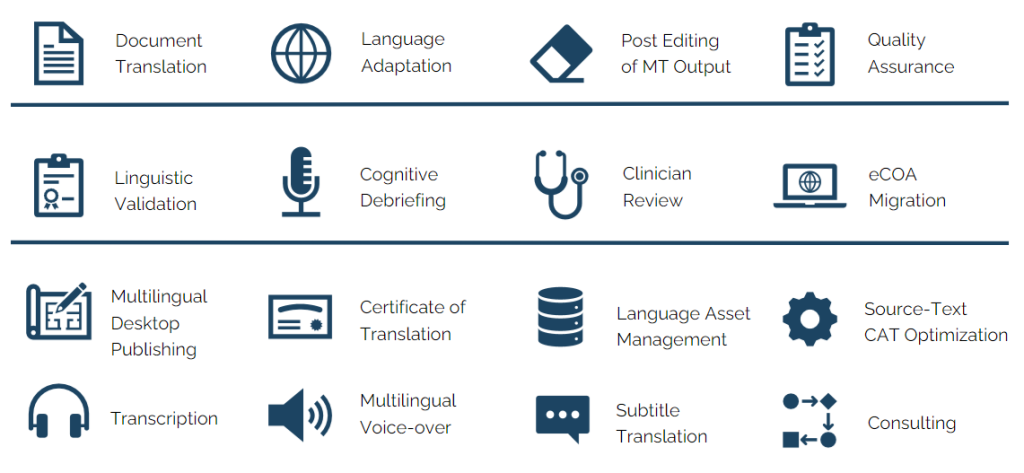
- Medical Devices Translations
- Medical Supplies & Disposables Translations
- In Vitro Diagnostics Translations
- Implantable Devices/Implant Translations
- Pharma Translations
- Clinical Trials Translations
- Regulatory Documents Translation
- Healthcare Translations
- Health/Fitness Translations
"Commit Global is the best company I’ve had the pleasure of working with for global translation services over the past 25 years. Every other company is second rate by comparison. 10/10 rating."
JustinCEO & PRESIDENT, MEDICAL DEVICES COMPANY
"I rely on Commit Global for translations of clinical documents. They have always come through with flying colors. Always on time, and with a professional product."
SharonVICE PRESIDENT. MARKET DEVELOPMENT AND CLINICAL AFFAIRS
"The right partner for medical technical translation services."
NikosCOMMERCIAL DIRECTOR, MEDICAL EQUIPMENT COMPANY
"I've been utilizing Commit for translation services for almost 5 years and have had nothing but positive experiences. They've always surprised me with their swift response time, and have saved me on more than a few occasions when I needed to turn something around quickly. They have proven to be a reliable and valuable supplier partner. Highly recommend!!"
DarrylynPRODUCT MANAGER, MEDICAL DEVICE COMPANY
"I have been working with Commit Global for over 5 years now and they continue to impress. The customer service we have received has been nothing less than stellar."
DeanaSENIOR CLINICAL RESEARCH ASSOCIATE, MEDICAL DEVICE COMPANY
"Commit's responsiveness and partnering are best in class!"
TreasureSR. MANAGER, HEALTHCARE/MEDICAL DEVICE COMPANY
Previous
Next
Language Services for Life Sciences